| Author |
 Topic Topic  |
|
|
kkrize
3 Posts |
 Posted - 02/22/2014 : 18:13:01 Posted - 02/22/2014 : 18:13:01


|
I am hoping what I experienced might help someone else. I have had multiple skin cancers - both squamous and basal cell. It all started several years ago with the first lesion on my left leg just above the heel. I had surgery to remove it. I subsequently found more lesions which appeared month after month for more than a year. More surgeries (5 total) on my left leg, chest, left arm. My left calf is riddled with scars.
I started treating lesions myself with blood root for the larger ones and hydrogen peroxide for the smaller ones. I was freaking out! I then developed a very aggression lesion on my face just under my right eye. I was very fearful of a deforming surgery so treated it myself with blood root. I could not leave the house for 6 days and experienced much pain and swelling but, amazingly, it all healed with barely a scar but it took 2 treatments and a trip to my dermatologist.
The reason I am relating this story is because I was convinced the sun was not the reason I was getting so many skin cancers. I felt there was a trigger. I searched the internet for answers. I reviewed my diet and supplements, I discussed causes with my dermatologist. I went down blind alleys. Then one day I typed in a search for fish oil and a link to skin cancer. Bingo! I found some articles that led to more information and then even more information. I discovered there was controversy over the much hyped benefits of fish oil. I had bought into the myths and had been taking a supplement, along with consumption of salmon and sometimes flaxseed in my smoothies for years. I stopped all consumption of fish oil, fatty fish and flaxseed oils. I also started to be careful about all PUFA consumption and only used coconut oil or butter for cooking and olive oil on my salads. The skin cancers ceased! I have not had another skin cancer for over a year now! I am convinced the increased use of fish oils corresponds to the alarming increase in skin cancers around the world. Take a look at this article: http://www.hindawi.com/journals/jl/2014/495761/. . . specifically points 22, 23. There seems to be a possible link to other cancers as well.
|
Edited by - kkrize on 02/22/2014 18:30:19 |
|
|
kkrize
3 Posts |
|
|
gloe
127 Posts |
 Posted - 06/04/2014 : 09:30:17 Posted - 06/04/2014 : 09:30:17


|
I find this to be very interesting and disturbing, especially because my doc has me on a high dose of Omega-3 oils. Still I am glad to hear of your experience with withdrawing the use of omega-3 oils. I may experiment also and see what happens.
Still, I have a couple of comments. First, section 22.2, it is reported that "Meticulous study (confirmed by pathology and cancer registry) of over 50,000 Norwegian men and women showed approximately a 3-fold increase in melanoma in women using cod liver oil (considered a superb fish oil supplement)." My doc says that cod liver oil is NOT a good fish oil supplement because it contains alot of vitamin A, and that has been shown to cause cancer in excess, IIRC.
Second, the increased incidence of skin cancers in Australia, Scandinavia, Canada and the US -- "Given that people are in the sun less and use sunscreen more, there are few valid reasons why skin cancer rates should be increasing worldwide and, in particular, these countries." The author attributes the increase to the use of fish oil supplements. However, it is controversial whether moderate sun exposure causes skin cancer to begin with; avoiding the sun leads to deficiencies in vitamin D which is an essential nutrient and may play a role in cancer. Also, commercial sunscreens are loaded with toxic chemicals and they may themselves promote skin cancer.
I find this very interesting in section 24, Discussion: "However, there are many cultures with excellent health and longevity that do not consume fish—the Hunza in Pakistan, the Vicambamba high in the Andes in Ecuador, the Abhasia of the Caucasus Mountains, and—in the United States—fully vegetarian 7th Day Adventists." Having been recently researching "vitamin" B17, I know the Hunza are famous for their apricot seed consumption, and I would imagine that people living in somewhat primitive conditions, as well as vegetarians, have a diet high in amygdalins from grains, nuts and seeds.
As for anecdotes, my father has been diagnosed with metastatic melanoma. He loves fish and eats a ton of it. He also has had aggressive prostate cancer. http://healthyliving.msn.com/diseases/cancer/too-much-fish-oil-might-boost-prostate-cancer-risk-study-says
Correlation never proves causation, but these ideas about fish oils are enough to get me to lay off for a while. Thanks for posting.
|
Edited by - gloe on 06/04/2014 09:59:20 |
 |
|
|
dan
611 Posts |
 Posted - 06/04/2014 : 20:50:39 Posted - 06/04/2014 : 20:50:39


|
Thanks to both of you for your posts. We tend to think of omega 3's as the good and omega 6's as the bad polyunsaturated fats. Maybe there is not much good in consuming too much of either. The rise in polyunsaturated fats in the diet have translated to a rise in polyunsaturated fats in the skin. This correlates well with a rise in skin cancer incidence. The underlying idea is that polysaturated fat is unstable and is easily damaged in skin structures.
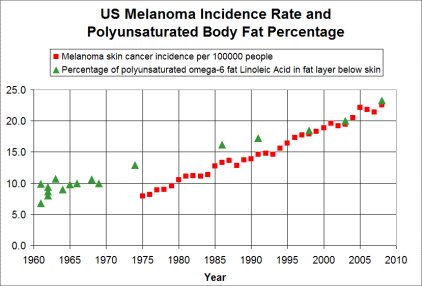
I'll probably stop my supplementation of fish oil too. There has not been much benefit shown in studies from omega 3's for heart health. The only reason to keep supplementing fish oil is for a potential benefit to brain health. |
 |
|
|
gloe
127 Posts |
 Posted - 06/04/2014 : 23:29:45 Posted - 06/04/2014 : 23:29:45


|
Thanks Dan. Come to think of it, my skin problem has gotten worse since I "gave in" to my doc's urging to increase my omega-3 intake. I am done with it for now. Reading that article I was thinking about my father's diet and all his cancer problems: Aggressive prostate cancer; many BCC, SCC (just like kkrize reported in himself), and now metastatic melanoma. I don't eat fish (never liked it), but have been taking Nordic Naturals Omega-3 by the tablespoon!
|
 |
|
|
anivoc
669 Posts |
 Posted - 06/12/2014 : 14:46:03 Posted - 06/12/2014 : 14:46:03


|
A healthy dose of skepticism is always a good way to start with analyzing data and information...Google "Lying with statistics"..
If Peskin is on to something genuine... awesome...it would be fantastic if all we had to do is change what we eat and all cancer on everyone goes away...It would be a true God send...I'm just VERY Skeptical of this guy...
I don't know either way on the fish oil..jury is still out for me..but to put it in perspective read this article debunking the study that fish oil increases the risk of or causes prostate cancer...
http://www.naturalnews.com/041344_fish_oil_omega_3_junk_science.html
In regards to the hindawi link mentioned above the author (Brian Peskin) has less than a spotless history...
He sells stuff..supplements ...books...fad diet pills...he's been shut down by the State of Texas.. ( to be fair the government shuts down good guys Like Greg Caton all the time too) I'm not a fan of Brian from what I have read so far...He has falsely advertised and lied about his education. To me it appears has an agenda and it is money motivated...that throws a ton of extra skepticism into my analysis of anything this guy is saying.. His latest greatest book is on PEO's (parent essential oils)and guess what...in the end it's all about selling his stuff...yep http://www.peskinpharma.com/
So before y'all stop eating fish and taking omega3's do your own due diligence...
Read # 12 & 13 below...
Texas Attorney General's Complaint against Brian Peskin
On April 26, 2002, the Attorney General of Texas issued this complaint charging Brian S. Peskin and his company with making misleading claims about his credentials and three Radiant Health products they were marketing. In January 2003, the District Court issued a permanent injunction ordering Peskin and his company to pay $100,000 to the State of Texas and to refrain from making a long list of unsubstantiated claims about their Radiant Health Products and Peskin's credentials.
STATE OF TEXAS,
Plaintiff
v.
PERKINS MANAGEMENT, INC.
D/B/A MAXIMUM EFFICIENCY
PRODUCTS & BRIAN SCOTT PESKIN
Defendant
§
§
§
§
§
§
§
§
§
IN THE DISTRICT COURT
HARRIS COUNTY
55th District Court
No. 2002-21594
PLAINTIFF'S ORIGINAL PETITION AND APPLICATION
FOR TEMPORARY AND PERMANENT INJUNCTION
TO THE HONORABLE JUDGE OF SAID COURT:
Comes now, the State of Texas ("Plaintiff"), acting by and through Attorney General John Cornyn, complaining of Brian Scott Peskin, Individually, and Perkins Management, Inc., dba Maximum Efficiency Products. Plaintiff would respectfully show the Court the following:
DISCOVERY ELECTION
1. Pursuant to Texas Rule of Civil Procedure 190.1, Plaintiff designates its intent to conduct discovery under Level 2 in compliance with Texas Rule of Civil Procedure 190.3.
JURISDICTION
2. This action is brought by Attorney General John Cornyn, through his Consumer Protection Division, in the name of the State of Texas and in the public interest under the authority granted him by §§ 431.047 and 431.0585 of the Texas Food, Drug and Cosmetic Act, TEX. HEALTH & SAFETY CODE ANN. § 431.001 et seq. ("TFDCA") upon the ground that the Commissioner of Health of the State of Texas and his authorized agents find that Defendants have violated provisions of § 431.021 of the TFDCA.
3. This action is also brought by Attorney General John Cornyn through his Consumer
Protection Division in the name of the State of Texas under the authority granted to him pursuant to § 17.47 of the Texas Deceptive Trade Practices Act, TEX. BUS. & COM. CODE ANN. § 17.41 et seq., ("DTPA") upon the grounds that Defendants have engaged in false, misleading and deceptive acts and practices in the conduct of trade or commerce as defined and declared unlawful by § 17.46 (a) and (b) of the DTPA.
PARTY DEFENDANTS
4. Defendant Perkins Management, Inc., dba Maximum Efficiency Products, is a Texas Corporation who may be served with process by serving its registered agent Stephen Peskin at 2922 Corder Street, Houston, Texas 77054.
5. Defendant Brian Scott Peskin is an individual residing in Harris County, Texas and who may be served with process at 2922 Corder Street, Houston, Texas 77054.
VENUE
6. Venue for this action lies in Harris County under the statutory authority of §§ 431.0585 and 431.047(c) of the TFDCA because the violations occurred in Harris County, Texas. Venue for this action also lies in Harris County pursuant to § 17.47 (b) of the DTPA because Defendants reside and have their principal place of business in Harris County, Texas. Venue also lies in Harris County, Texas pursuant to § 15.002 (a)(1)-(3) TEX. CIV. PRACT. & REM. CODE because Harris County is the place where all or a substantial part of the events or omissions giving rise to the claim occurred, and because both the individual Defendant's residence and the Corporate Defendant's principal office were situated in Harris County at the time this cause of action accrued.
PUBLIC INTEREST
7. Because Plaintiff State of Texas has reason to believe that Defendants have engaged in, and will continue to engage in the unlawful practices set forth below, Plaintiff State of Texas has reason to believe Defendants have caused and will cause immediate, irreparable injury, loss and damage to the State of Texas, and will also cause adverse effects to legitimate business enterprises which lawfully conduct trade and commerce in this State. Therefore, the Consumer Protection Division of the Office of the Attorney General of the State of Texas believes and is of the opinion that these proceedings are in the public interest.
TRADE AND COMMERCE
8. Defendants have, at all times described below, engaged in conduct constituting "trade" and "commerce," as those terms are defined in §17.45(6) of the DTPA.
ACTS OF AGENTS
9. Whenever in this petition it is alleged that a Defendant did any act, it is meant that:
A. The Defendant performed or participated in the act, or
B. The Defendant's officers, agents, trustees or employees performed or participated in the act on behalf of and under the authority of the Defendant.
NOTICE BEFORE SUIT
10. Pursuant to §17.47(a) of the Deceptive Trade Practices Act, contact has been made with the Defendants' counsel herein, to inform them of the unlawful conduct alleged herein and that this action would be filed.
NATURE OF DEFENDANTS' CONDUCT
11. Defendants manufacture, offer for sale, sell, distribute and advertise foods throughout the United States, England, Thailand, and other countries. Defendants manufacture the following three products under the trademarked name of Radiant Health: (1) a tonic called "Herbal Essence" which is an acidified food made of Burdock Root, Sheep Sorrel, Cat's Claw bark, Slippery Elm bark, and Turkish Rhubarb Root brewed with apple cider vinegar and water; (2) a nutritional supplement pill called "Basic Essence" which is made from various oils; and (3) a mineral pill called "Mineral Essence" which is made from various minerals.
12. Defendants' products are advertised primarily on television and via the Internet. Defendants' advertisements, including their promotional brochures, tapes, CD's, and pamphlets, make a number of false claims about the beneficial effects of the three Radiant Health products. Defendants claim that their Radiant Health products will: (1) help you lose weight; (2) boost your immune system; (3) protect against heart disease; (4) reduce the risk of breast, prostate and other cancers; (5) increase your energy, improve concentration, and minimize the harmful effects of stress; (6) eliminate varicose veins; (7) lower blood pressure; (8) lower cholesterol; (9) eliminate cellulite; (10) cure constipation; (11) prevent diabetes; (12) manage ADD; (13) eliminate fatigue; (14) reduce your appetite. Defendants also claim that their Radiant Health products are great for children, infants and pregnant or nursing mothers. Defendants claim that all their products help children with ADD, ADHD, and hyperactivity. Defendants even claim that their products make children smarter. These health and disease claims cannot be substantiated by Defendants, and are thus false, misleading and deceptive.
13. Defendants also advertise extensively that their products were created by Defendant Brian Scott Peskin and his team of "Life-Systems scientists." Defendants falsely represent that Brian S. Peskin is the "Holder [of the] Emeritus Life-Systems Engineering Chair, College of Pharmacy and Health Sciences, Texas Southern University." Defendants also falsely represent that Brian Peskin is a doctor, scientist, and professor. Defendants fail to disclose that Brian Peskin has a degree in electrical engineering. The representations that expressly and impliedly exaggerate the credentials and expertise of Brian Peskin are false, misleading and deceptive in that they have the tendency to deceive the buying public. Defendants use of Brian Peskin's book ("Radiant HealthMoving Beyond the Zone), cassette tapes, video tape, and CD's, together with other promotional materials, brochures, pamphlets, and various Internet websites creates and facilitates widespread false advertising of Defendants' products, as these materials contain false, misleading and deceptive representations.
14. Defendants failed to file and register as a commercial processor of acidified foods ("Herbal Essence") with the U.S. Food and Drug Administration ("FDA"), despite manufacturing such food for years. This failure to file and register violates the State and Federal Food & Drug Acts. Defendants were required by law to have so registered and filed within 10 days of engaging in the manufacture, processing or packing of acidified foods. Defendants also failed to provide the FDA with detailed information on the scheduled manufacturing process of their "Herbal Essence" tonic or tea, which was required by law to be filed not later than 60 days after they [should have] initially registered with the FDA. Defendants failed to promptly report to the FDA any instances of spoilage, process deviation, or contamination with microorganisms for "Herbal Essence" being distributed in commerce covering the period from 1993 to the time of filing this suit. Defendants failed to prepare, review and retain at their processing plant, for a period of three years from date of manufacture, all records of processing, deviations in processing, pH, calibration, packaging, and other necessary records relating to the processing of acidified foods. Defendants' failure to follow appropriate quality control operations to ensure that the "Herbal Essence" product was safe and suitable for human consumption raises serious issues about the integrity of their entire manufacturing process. Defendants failed to maintain processing and production records showing adherence to scheduled processes, including records of pH measurements and other critical factors intended to ensure a safe product. Acidified foods with pH levels higher than 4.6 allow harmful microorganisms to produce which can be harmful to the end user.
15. All of Defendants' products (Herbal Essence, Basic Essence & Mineral Essence) are misbranded because: (1) their labeling does not include the following FDA disclaimer statement for structure/function claims: "This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease;" (2) the "heart smart" vignette displayed on the labeling and advertising materials implies an unsubstantiated and ineligible health claim which has not been substantiated by Defendants or validated or approved by the FDA; (3) the labeling and advertising materials expressly represent that these products are safe, effective, and specifically intended for infants and toddlers less than two years of age, which is prohibited; (4) the labeling and advertising materials expressly and impliedly represent that Defendants' products will mitigate, treat, cure, or prevent specific diseases and classes of diseases as well as performing a role in the body's response to a disease which subjects such products to regulation as a drug; (5) they are labeled and advertised as drugs without FDA approval; (6) the principal display panel of Defendants' product packaging fails to state a common or usual name of the food product in that the name given the product does not describe the nature of the product; and (7) the serving size on the labeling of the Basic Essence and Herbal Essence "Nutrition Facts" panel differs from the serving size on the information panel; (8) they are not labeled as dietary supplements; and (9) the labeling sets forth a "Nutrition Facts" panel instead of a "Supplement Facts" panel as required by law.
16. On March 8, 2002, The Commissioner of Health issued a Recall Order requiring Defendants to recall all Radiant Health "Herbal Essence" products which were distributed in commerce. The recall was issued because Defendants' products were produced, prepared, packed and held whereby they may have been rendered injurious to health. Defendants only partially complied with this Recall Order and failed to recall products shipped overseas as well as products shipped directly to consumers. These failures allowed misbranded and adulterated food products to remain in commerce against the direct orders of the Commissioner of Health.
VIOLATIONS OF THE TEXAS FOOD, DRUG AND COSMETIC ACT
17. Defendants have engaged in the following prohibited acts, as alleged in this petition, which violate the TFDCA:
A. The introduction or delivery for introduction into commerce of any food or drug that is adulterated or misbranded in violation of TFDCA §431.021(a);
B. The adulteration or misbranding of any food or drug in commerce in violation of TFDCA §431.021(b);
C. The distribution in commerce of a consumer commodity, if such commodity is contained in a package or has labeling that does not conform to the provisions of Chapter 431 of the TFDCA, in violation of TFDCA §431.021(d);
D. The dissemination of false advertisements, as alleged herein, in violation of TFDCA §431.021(f);
E. The manufacture within this state of any food, drug, device, or cosmetic that is adulterated or misbranded in violation of TFDCA §431.021(h);
F. Engaging in the manufacture of food in Texas without first being duly licensed with the Department of Health as required by TFDCA §431.222.
G. Failing to submit a scheduled process for production of Defendants' "Herbal Essence" tonic, which is an acidified food, as well as to register with the FDA in violation of TFDCA §431.244(a) which adopts 21 C.F.R. 108.25 (c)(1)-(2);
H. Failing to prepare, review and maintain, for a period of (3) years at Defendants' processing plant, all records of processing, deviations in processing, pH and other records specified in 21 C.F.R. 114 in violation of TFDCA §431.244(a) which adopts 21 C.F.R. 108.25(g) and 21 C.F.R. 114;
I. Failing to maintain processing and production records showing adherence to scheduled processes, including records of pH measurements, calibration of all instruments used in the process, and records of other critical factors intended to ensure production of a safe product, in violation of TFDCA §431.244(a) which adopts 21 C.F.R. 114.100(a)-(e);
J. Distributing in commerce any acidified foods with a finished equilibrium pH value of 4.3 or higher achieved within the time designated in the scheduled process and maintained in all finished foods in violation of TFDCA §431.244(a) which adopts 21 C.F.R. 114.80(a)(1).
K. The introduction or delivery for introduction into commerce any new drug not approved by the FDA as required by TFDCA §431.114, in violation of TFDCA §431.021(e).
VIOLATIONS OF THE DTPA
18. Defendants, in the course and conduct of trade and commerce, have directly and indirectly engaged in false, misleading and deceptive acts and practices declared to be unlawful by the DTPA, §17.46(a) and DTPA, §17.46(b), to wit:
A. Causing confusion or misunderstanding as to the source, sponsorship, approval, or certification of goods or services, in violation of DTPA, §17.46(b)(2);
B. Causing confusion or misunderstanding as to affiliation, connection, or association with, or certification by, another, in violation of DTPA, §17.46(b)(3);
C. Representing that goods or services have sponsorship, approval, characteristics, ingredients, uses, benefits, or quantities which they do not have, or that a person has a sponsorship, approval, status, affiliation, or connection which he does not have, in violation of DTPA, §17.46(b)(5);
D. Representing that goods or services are of a particular standard, quality, or grade, or that goods are of a particular style or model, if they are of another, in violation of DTPA, §17.46(b)(7);
E. Advertising goods or services with intent not to sell them as advertised, in violation of DTPA, §17.46(b)(9)
F. Failing to disclose information concerning goods or services which was known at the time of the transaction if such failure to disclose such information was intended to induce the consumer into a transaction which the consumer would not have entered had the information been disclosed, in violation of DTPA, §17.46(b)(24);
G. Engaging in false, misleading or deceptive acts or practices in the course of trade or commerce, in violation of DTPA § 17.46(a).
18.1 Defendants have, by means of the unlawful acts and practices described in this petition, obtained money or other property from identifiable persons to whom such money or property should be restored, or who in the alternative are entitled to an award of damages.
DISGORGEMENT
19. Defendants' assets are subject to the equitable remedy of disgorgement, which is the forced relinquishment of all benefits that would be unjust for Defendants to retain, including all ill-gotten gains and benefits or profits that result from Defendants fraudulent actions. Defendants should be ordered to disgorge all monies fraudulently taken from individuals and businesses together with all of the proceeds, profits, income, interest and accessions thereto. Such disgorgement should be for the benefit of victimized consumers and the State of Texas.
TRIAL BY JURY
20. Plaintiff herein requests a jury trial and tenders the jury fee to the Harris County District Clerk's office pursuant to TEX. R. CIV. P. 216 and TEX. GOV'T CODE ANN. §51.604.
APPLICATION FOR TEMPORARY INJUNCTION
AND PERMANENT INJUNCTION
21. Because Defendants have engaged in the unlawful acts and practices described above, Defendants have violated and will continue to violate the law as alleged in this Petition. Unless immediately restrained by this Honorable court, Defendants will continue to violate the laws of the STATE OF TEXAS and cause immediate, irreparable injury, loss and damage to the State of Texas and to the general public. Therefore Plaintiff requests a Temporary Injunction and Permanent Injunction as indicated below.
PRAYER
22. WHEREFORE, Plaintiff prays that Defendants be cited according to law to appear and answer herein; that after notice and hearing a TEMPORARY INJUNCTION be issued; and upon final hearing a PERMANENT INJUNCTION be issued, restraining and enjoining Defendants, Defendants' successors, assigns, officers, agents, servants, employees and attorneys and any other person in active concert or participation with Defendants, from engaging in the following acts or practices:
(1) Transferring, concealing, destroying, or removing from the jurisdiction of this Court any books, records, documents, invoices or other written materials relating to the business of Defendants currently or hereafter in Defendants' possession, custody or control except in response to further orders or subpoenas in this cause;
(2) Introducing or delivering for introduction into commerce any food or drug that is adulterated or misbranded in violation of TFDCA §431.021(a);
(3) Adulterating or misbranding any food or drug in commerce in violation of TFDCA §431.021(b)
(4) Distributing in commerce any consumer commodity, if such commodity is contained in a package or has labeling that does not conform to the provisions of Chapter 431 of the TFDCA;
(5) Manufacturing within this State any food or drug that is adulterated or misbranded within the meaning of Chapter 431 of the TFDCA;
(6) Engaging in the manufacture or processing of food products in Texas without first being properly licensed with the Department of Health as required by TFDCA §§ 431.222.
(7) Failing to completely comply with the scheduled process submitted and filed with the FDA for production of Defendants' "Herbal Essence" tonic;
(8) Failing to prepare, review and maintain, for a period of (3) years from date of manufacture, at Defendants' processing plant, all records of acidified food processing, records of deviations in acidified food processing, routine and required pH testing of acidified foods, temperature (hot fill) records relating to acidified food production, acidified food batch records, records of required/routine calibration of all necessary instruments utilized in the processing of acidified foods, such as thermometers and pH meters, and acidified food distribution records, as required by TFDCA §431.244(a), 21 C.F.R. 114, & 21 C.F.R. 108.25 (c)(1)-(2);
(9) Distributing any acidified food products, including, but not limited to Radiant Health Herbal Essence, with a finished equilibrium pH value of 4.3 or higher achieved within the time designated in any scheduled process submitted to the FDA, and to maintain such finished equilibrium pH value of 4.3 or lower in all finished foods;
(10) Processing or manufacturing any food product which is introduced into commerce which has been produced, prepared, packed or held by Defendants in any way whereby such product may have been rendered unwholesome or injurious to health as a result of any pH testing not in compliance with this Order or any deviation in processing from the scheduled process submitted to the FDA;
(11) Processing or manufacturing any food product which is introduced into commerce if the labeling on, or accompanying such product, is false or misleading in any particular as set forth in Chapter 431 of the TFDCA or any companion Texas Administrative Code provisions;
(12) Processing or manufacturing any food product which is introduced into commerce unless the label on, or accompanying such product, bears the common or usual name of the food, if any;
(13) Offering for sale, selling, or distributing into commerce any food product intended for human consumption if any claim is made on the label, or in any type of advertising related to the product, which expressly or impliedly refers to the nutrient content or a nutritional quality of the food product to a specific disease or condition of the human body, except as permitted by § 403(r) of the Federal Food and Drug Act;
(14) Offering for sale, selling, or distributing into commerce any food product intended for human consumption if any claim is made on the label, or in any type of advertising related to the product, which expressly or impliedly refers to the nutrient content of the product or to any health claims made by the product which is not in compliance with § 403(r) of the Federal Food and Drug Act;
(15) Advertising or labeling any food product or drug which makes any express or implied claims that such product will (1) help you lose weight; (2) boost your immune system; (3) protect against heart disease; (4) reduce the risk of breast, prostate and other cancers; (5) increase your energy, improve concentration, minimize the harmful effects of stress; (6) eliminate varicose veins; (7) lower blood pressure; (8) lower cholesterol; (9) eliminate cellulite; (10) cure constipation; (11) prevent diabetes; (12) manage ADD; (13) eliminate fatigue; and (14) reduce your appetite; (15) help children or other persons with ADD, ADHD, or hyperactivity; (16) be safe for infants, toddlers, or is beneficial in treating or preventing any type of disease or health related condition;
(16) Using any vignette or symbol, including the "heart smart" vignette, on any food or drug product advertised, manufactured, processed, sold, or distributed which makes any health or disease claim which has not been validated and approved by the FDA;
(17) Representing, expressly or by implication, in any labeling or advertising of food products, that such products will mitigate, treat, cure, or prevent specific diseases and classes of diseases, as well as performing any role in the human body's response to a disease, which subjects such products to regulations as a drug;
(18) Labeling and advertising products of any kind as drugs without prior FDA approval;
(19) Failing to properly label any food distributed into commerce, including, but not limited, to listing a "Nutrition Facts" panel in the proper format on the label as required by law;
(20) Listing different serving sizes on the labeling of Basic Essence and Herbal Essence products;
(21) Introducing or delivering for introduction into commerce any new drug without a new drug application being approved by the FDA as required by TFDCA §431.114;
(22) Distributing, selling, shipping, mailing, delivering, or sending any of Defendants' products (Basic Essence, Mineral Essence or Herbal Essence) to any person, entity, or business which uses any of the following (or similar) express or implied representations in any advertising by such person, entity or business (including the Internet) relating to Defendants' food products: that Defendants' products will (1) help you lose weight; (2) boost your immune system; (3) protect against heart disease; (4) reduce the risk of breast, prostate and other cancers; (5) increase your energy, improve concentration, and minimize the harmful effects of stress; (6) eliminate varicose veins; (7) lower blood pressure; (8) lower cholesterol; (9) eliminate cellulite; (10) cure constipation; (11) prevent diabetes; (12) manage ADD, ADHD or hyperactivity; (13) eliminate fatigue; (14) reduce your appetite; (15) are great for children, infants and pregnant or nursing mothers; (16) make children smarter; and (17) any other express or implied health or disease related claim which has not been substantiated by Defendants and approved by the FDA;
(23) Distributing, selling, shipping, mailing, delivering, or sending any of Defendants' food products (Basic Essence, Mineral Essence or Herbal Essence) to any person, entity, or business which uses any of the following (or similar) express or implied representations in any advertising (including the Internet) relating to the credentials, education, background or expertise of Brian Scott Peskin: (1) that Brian Scott Peskin is the "Holder [of the] Emeritus Life-Systems Engineering Chair, College of Pharmacy and Health Sciences, Texas Southern University;" (2) that Brian Scott Peskin is a doctor, scientist, professor or holder of a Ph.D.; and (3) any other representation that expressly or impliedly exaggerates the credentials, expertise, background or education of Brian Scott Peskin;
(24) Distributing, selling, shipping, mailing, delivering or sending any of Defendants'Herbal Essence to any person, entity or business that previously received any such product from October 1, 2001 through April 16, 2002, without notifying them in a separate written letter in 12 point font (and also orally in the event of a telephone or in person order) as follows: " We request that any Radiant Health Herbal Essence tonic which may have been shipped to you in the last 6 months be returned to us for either a full refund, credit or replacement. Unfortunately, the Herbal Essence tonic shipped to customers during this time was not processed with normal quality controls, and in order to maintain the highest quality of the product, we request a return of the product for a full refund, credit or replacement. Please call us if you have any questions, or if you would like to dispose of the unused product instead of mailing it to us."
(25) Selling, distributing, sending, mailing, printing, giving, disseminating, advertising,or allowing any other person, entity or business to sell, distribute, send, give, mail, print, advertise, or disseminate the book "Radiant HealthMoving Beyond the Zone" to any person, entity or business, at any time in which Defendants or others are producing, manufacturing, distributing, selling, giving, sending, advertising or marketing any of Defendants' current products (now) known as Radiant Health Herbal Essence, Mineral Essence and/or Basic Essence;
(26) Including health claims on the label or in labeling that expressly or by implication characterize the relationship of any substance in a food product to a disease or health-related condition other than those which are complete, truthful and not misleading in regard to the product and have been approved by the FDA;
(27) Representing, expressly or by implication, in any advertising of any product, that Defendant Brian Scott Peskin is a "scientist," "Professor," or "Doctor," or that Brian Scott Peskin is the "holder of the Emeritus Life-Systems Engineering Chair, College of Pharmacy and Health Sciences at Texas Southern University;"
(28) Representing, expressly or by implication, in any type of advertising of any food or drug product, that Brian Scott Peskin is an MIT engineer, unless it is affirmatively disclosed in said advertising that such engineering degree was in electrical engineering;
(29) Exaggerating, expressly or by implication, the credentials, expertise, or educational background of Brian Scott Peskin or any other person, employee or agent of Defendants associated in any way with the manufacture, production, marketing, distribution, or sale of any food or drug related product;
(30) Facilitating, assisting, consenting, or requesting any person or entity to engage in any advertising of Defendants' products which uses any of the express, implied (or similar) representations to which Defendant is prohibited from engaging in; and
(31) Including descriptive claims for a dietary supplement on the label or in labeling unless Defendants have substantiation that the statements are truthful and not misleading.
22.1 In addition, Plaintiff STATE OF TEXAS respectfully prays that this Court will:
A. Adjudge against Defendants civil penalties in favor of Plaintiff STATE OF TEXAS in an amount up to $10,000 per violation, not to exceed $100,000.00 allowed by law under the DTPA, specifically, §17.47(c)(2) of the Texas Business and Commerce Code, due to Defendants committing acts and practices which were calculated to acquire or deprive money or other property from consumers who were 65 years of age or older when the act or practice occurred;
B. Adjudge against Defendants civil penalties in favor of Plaintiff, STATE OF TEXAS, in an amount up to $2,000 per violation, not to exceed $10,000 allowed by law under the DTPA, specifically, §17.47(c)(1) of the Texas Business and Commerce Code;
C. Adjudge against Defendants civil fines and penalties to the State of Texas in the amount of $25,000 per violation per day, for each violation of TFDCA §431.021 as provided in TFDCA §431.0585;
D. Order Defendants to restore all money or other property taken from identifiable persons by means of unlawful acts or practices, or, in the alternative, award judgment for damages1 in an amount within the jurisdictional limits of this court to compensate for such losses;
E. Order Defendants to pay Plaintiff STATE OF TEXAS attorney fees, expenses, and costs of court pursuant to TEX. GOV'T CODE ANN. §402.006(c) and TEX. HEALTH & SAFETY CODE ANN. §§161.094(d) and 431.047(d);
F. Order the disgorgement of all sums taken from consumers by means of deceptive trade practices, together with all proceeds, interest, income, profits and accessions thereto;
G. Grant all other relief to which the Plaintiff, State of Texas, may show itself entitled.
Respectfully submitted,
JOHN CORNYN
Attorney General of Texas
HOWARD BALDWIN, JR.
First Assistant Attorney General
JEFFREY BOYD
Deputy Attorney General for Litigation
PAUL CARMONA Chief, Consumer Protection Division
JOHN OWENS
Assistant Attorney General
Consumer Protection Division
808 Travis, Suite 812
Houston, Texas 77002
713/223-5886, ext. 218
713/223-5821 (fax)
State Bar No. 15379200
SPIN NO. 99999928
VERIFICATION
STATE OF TEXAS
COUNTY OF HARRIS
Before me, the undersigned Notary Public, on this day personally appeared Dana Cotton, who, after being duly sworn, stated under oath that he is employed by the Texas Department of Health in this action, that he has read the above petition, and that every factual statement contained in the petition is true and correct and within the personal knowledge of affiant.
Dana Cotton
SUBSCRIBED AND SWORN TO BEFORE ME, on the 26th day of April 2002, to certify which witness my hand and official seal.
NOTARY PUBLIC
State of Texas
|
Edited by - anivoc on 06/12/2014 16:26:20 |
 |
|
|
blue
11 Posts |
 Posted - 03/05/2017 : 01:28:49 Posted - 03/05/2017 : 01:28:49


|
| Fishy oil really works for skin cancer. A study by scientists at the Fred Hutchinson Cancer Research Center in Seattle linked eating a lot of oily fish or taking potent fish oil supplements to a 43% increased risk for prostate cancer overall, and a 71% increased risk for aggressive prostate cancer. In addition, omega-3 fatty acids found in the oil play important roles in brain function, normal growth and development, and inflammation. |
 |
|
|
dan
611 Posts |
 Posted - 03/10/2017 : 00:29:39 Posted - 03/10/2017 : 00:29:39


|
Hi blue, did you mean to say fish oil caused a decreased or increased risk of prostate cancer? It sounds like you are saying fish oil is good but a 43% increased risk for prostate cancer would, of course, be very bad. Can you share your sources in a link?
While in general what works to prevent one type of cancer will also benefit other types of cancers, a prostate cancer benefit for omega 3's may not carry over to skin cancers. This is because polyunsaturated fats (including omega 3 and omega 6 fats) in the diet become integrated in skin tissues, displacing saturated fats. (You are what you eat!) Saturated fats are very stable and are much less prone to damage by ultraviolet light compared to polyunsaturated fats.

This gets back to the three pillars of cancer prevention:
1. Minimize cell damage. (Injuries, UV light in the case of skin cancer.)
2. Optimize the immune system response to cancer. (Protein dissolving enzymes and natural killer cells circulating in the blood.)
3. Reduce cancer growth factors. (Good: vitamin D, melatonin, love. Bad: stress, estrogen, sugar)
In the prostate, the danger to damage from ultraviolet light is minimal. So omega 3's may positively influence the immune system response to cancer and/or reduce the cancer growth factors, but the cell damage factor may overwhelm those other 2 of the 3 pillars for skin cancer. If a person wants to benefit from omega 3 supplementation, they should probably also supplement antioxidants as well. In the case of fish oil supplementation, astaxanthin is an ideal supplement to take along with it.
Avoiding ultraviolet light is not ideal because that reduces the opportunity to naturally generate vitamin D, a potent anti-cancer hormone. Moderate and frequent sun exposure, maintaining healthy relationships, daily exercise, less stress, adequate rest, less processed food with more saturated fat, less omega 6 fat, less sugar, and less protein (especially before bedtime) in the diet, along with selectively using nutritional supplements seem to be the best approaches to me to avoid cancers of all types. |
 |
|
|
fRomance
81 Posts |
 Posted - 08/18/2021 : 21:27:02 Posted - 08/18/2021 : 21:27:02


|
| That's why I only get take some little amount of fish oil from eating fish instead of taking supplements, just a little amount and not even taking daily wouldn't hurt. |
 |
|
| |
 Topic Topic  |
|
|
|

